Effect of surface acidity of cyano-bridged polynuclear metal complexes on the catalytic activity for the hydrolysis of organophosphates†
Abstract
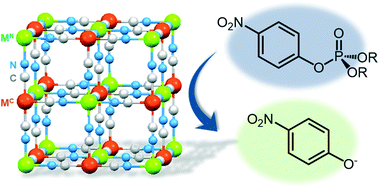
Heterogeneous catalysis of cyano-bridged polynuclear metal complexes, which were prepared by systematic replacement of C-bound metal ions (MC) and/or N-bound metal ions (MN) of Prussian blue ([MN(H2O)x]y[FeII/III(CN)6]; MN = FeIII, GaIII, MnII, ZnII or CoII: [FeII/III(H2O)x]y[MC(CN)6]; MC = FeII, PtIV, CoIII, IrIII or RuII), was examined for the hydrolysis of p-nitrophenol phosphate (p-NPP) as a model compound of insecticides. The catalytic activity of the complexes was enhanced by employing metal ions in higher oxidation states at the C- and N-bound sites, although only N-bound metal ions act as active sites. The dependence of the initial reaction rates for the hydrolysis on the initial concentration of p-NPP suggested that the rate determining step is the adsorption of p-NPP onto catalyst surfaces. The surface acidity of each complex estimated by the heat of pyridine desorption is strongly correlated with catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...