Precursor controlled synthesis of graphene oxide supported iron catalysts for Fischer–Tropsch synthesis†
Abstract
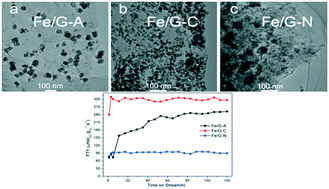
In this study, graphene oxide supported iron catalysts have been successfully synthesized by a hydrothermal method using three different iron precursors: ferrous acetate Fe(C2H3O2)2, ferric oxalate Fe2(C2O4)3 and ferric nitrate Fe(NO3)3. The Fe2(C2O4)3 derived catalysts (Fe/G-C) present a uniform dispersion with smaller sizes of iron nanoparticles (NPs). Density functional theory (DFT) calculations indicate the higher binding energy between ferric oxalate and graphene oxide (−1.53 eV) facilitates the formation of more seeds for nanoparticle growth, and therefore, smaller size and narrow size distribution of NPs are achieved. The Fe/G-C also exhibits easier reduction and carburization, resulting in high Fischer–Tropsch synthesis (FTS) activity and C5+ selectivity. Our thorough characterization studies enable us to conclude that the iron precursors significantly impact the structural properties of catalysts, which further affect their reduction/carburization abilities and FTS performances. This study affords us a rational design of high efficiency graphene oxide supported iron catalysts for FTS.


 Please wait while we load your content...
Please wait while we load your content...