The application of a nickel(ii) Schiff base complex in water oxidation: the importance of nanosized materials†
Abstract
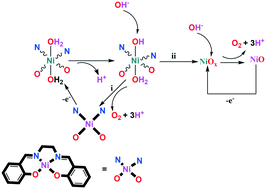
Herein, the role of Ni oxide in the electrocatalytic water oxidation of a nickel(II) Schiff base (N,N′-bis (salicylidene)ethylenediamino nickel(II)) is investigated using scanning electron microscopy, transmission electron microscopy, energy dispersive spectrometry, X-ray diffraction, nuclear magnetic resonance spectroscopy, extended X-ray absorption fine structure, X-ray absorption near edge structure, chronoamperometry, electrochemical methods and theoretical calculations. Although Ni oxide does not have a pure, simple and crystalline structure, the experiments showed that Ni oxide at a pH of 11 is a water-oxidizing catalyst on the surface of a fluorine-doped tin oxide electrode. At pH 3 or 7, no clear water oxidation or NiOx formation was detected. Below this low pH, a solid with high carbon content was detected, which is not a water-oxidizing catalyst. A metal oxide-based mechanism for water oxidation in the presence of a metal complex has not been considered using theoretical calculations. Herein, our theoretical calculations indicated that after the oxidation of the Ni(II) complex, all Ni equatorial bonds in the oxidation state of III were weakened. This effect may cause the decomposition of the intermediate to NiOx. This study could be very promising for the design and synthesis of new, efficient and stable catalysts.


 Please wait while we load your content...
Please wait while we load your content...