Mn(i) organometallics containing the iPr2P(CH2)2PiPr2 ligand for the catalytic hydration of aromatic nitriles†
Abstract
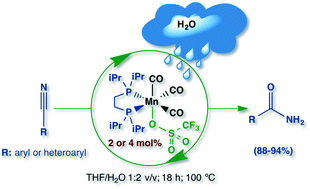
The first example of a homogeneous hydration of aromatic nitriles catalyzed by manganese molecular compounds is reported. The Mn(I) organometallics fac-[(CO)3Mn(dippe)(Z)]1−nX1−n (n = 0, 1; Z = Br, OTf, PhCN; X = OTf) were synthesized and characterized, and their reactivity was studied. The species fac-[(CO)3Mn(dippe)(OTf)] (2) was used as a catalyst precursor for the selective hydration of benchmark benzonitrile (2 mol% 2, THF/H2O 1 : 2 v/v, 18 h, 100 °C) to produce benzamide in 90% isolated yield. A series of (hetero)aromatic nitriles were hydrated to synthesize the corresponding amides in very good to excellent yields (88–94%). Isotopic labeling studies accounted for a proton transfer as the rate-determining step.


 Please wait while we load your content...
Please wait while we load your content...