Effect of various structure directing agents (SDAs) on low-temperature deactivation of Cu/SAPO-34 during NH3-SCR reaction†
Abstract
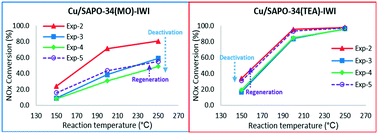
Cu/SAPO-34 and Cu/SSZ-13 having chabazite structure (CHA) have attracted significant attention because of their high activity and N2 selectivity during SCR reaction as well as superior resistance to hydrocarbon poisoning. Cu/SAPO-34 has shown better hydrothermal durability than Cu/SSZ-13 at high temperature. However, we have observed earlier that Cu/SAPO-34 prepared using morpholine as a structure directing agent (SDA) deteriorated under water exposure at low temperature, where the NOx conversion activity decreased from 87% to 6% after 9 h of low temperature exposure. In this study, Cu/SAPO-34 catalysts prepared using three different SDAs, i.e., morpholine (MO), triethylamine (TEA), and tetraethylammonium hydroxide (TEAOH), were prepared by the incipient wetness impregnation (IWI) method. A commercially purchased SAPO-34 (SAPO-34(ACS)) was also used for comparison purposes. After low temperature water deactivation, Cu/SAPO-34(TEA) and (TEAOH) mostly recovered their activities while Cu/SAPO-34(MO) and (ACS) only regained part of their activities after regeneration tests under a series of experimental conditions for the NH3-SCR reaction. Solid-state MAS NMR was employed to study the impact of SDAs on the coordination of Al, P, and Si in the SAPO-34 supports and Cu/SAPO-34 catalysts. CO-DRIFTS, NO-DRIFTS, and H2-TPR employed in this study collectively propose the presence of two different Cu locations in Cu/SAPO-34(MO, TEA, TEAOH, and ACS). It is suggested that the concentrations of Cu in two distinct locations within Cu/SAPO-34 catalysts characterized by CO-DRIFTS, NO-DRIFTS, and H2-TPR studies are significantly influenced by the choice of SDA, which will be important for understanding the deactivation mechanism of Cu/SAPO-34 catalysts during low temperature NH3-SCR reaction.


 Please wait while we load your content...
Please wait while we load your content...