Fluorine-enhanced Pt/ZSM-5 catalysts for low-temperature oxidation of ethylene†
Abstract
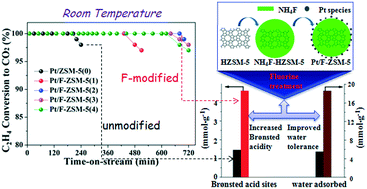
The low-temperature oxidation of ethylene (C2H4) is of critical importance in some specific places and environments such as refrigeration storage, but the catalyst stability needs to be further improved. The microporous Pt/ZSM-5 catalyst prepared in this study exhibited excellent catalytic performance in C2H4 oxidation. The modification of F on Pt/ZSM-5 was further investigated to explore the possibility of enhancing the catalytic performance for low-temperature oxidation of ethylene. Compared with unmodified Pt/ZSM-5 catalyst, the F modification has efficiently improved the reaction stability of the Pt/ZSM-5 catalyst. The conversion of C2H4 over 0.5% Pt/F-ZSM-5 could be sustained at 100% for more than 11 h at 25 °C. This study showed that the acidity and water tolerance of catalysts were crucial factors that affected the catalytic stability. The enhancement of the Brønsted acidity of the F-treated ZSM-5 was favourable for the adsorption and activation of C2H4 molecules onto the Pt/F-ZSM-5 catalysts. The F modification prolonged the catalyst life by improving its water tolerance. Thus, Pt/F-ZSM-5 has the potential to be an efficient low-temperature catalyst for C2H4 elimination in possible applications.


 Please wait while we load your content...
Please wait while we load your content...