Bimetallic Ni–Co catalysts supported on Mn–Al oxide for selective catalytic CO hydrogenation to higher alcohols†
Abstract
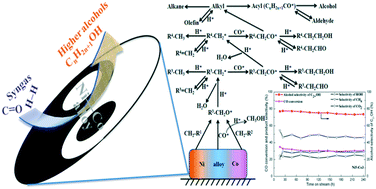
A series of bimetallic Ni–Co catalysts supported on Mn–Al oxide with different Ni/Co molar ratios were synthesized using a sol–gel method and applied for CO hydrogenation to higher alcohols. The physical and chemical properties of the as-prepared catalysts were investigated by various characterization methods such as XRD, N2 adsorption/desorption, H2-TPR, TEM, XPS, CO-TPD, H2-TPD, and CO-TPSR. The results indicated that a stronger interaction between Ni and Co ions led to the formation of a Ni–Co alloy in the reduced catalysts, and CO conversion and alcohol selectivity changed depending on the Ni/Co molar ratio that significantly influenced the properties of the catalysts. The appropriate Ni/Co molar ratio can improve the reducibility, increase the amount of non-dissociated CO on the catalyst surface for CO insertion, and enhance the catalytic performance for higher alcohol synthesis. In particular, a Ni/Co molar ratio of 5/3 was found to be the most suitable for the physical–chemical and catalytic properties of the bimetallic Ni–Co catalysts for CO hydrogenation to higher alcohols.


 Please wait while we load your content...
Please wait while we load your content...