Mechanistic study on –C–O– and –C–C– hydrogenolysis over Cu catalysts: identification of reaction pathways and key intermediates†
Abstract
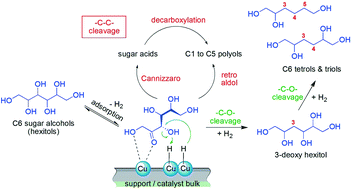
Important petro-based polyol compounds with a longer carbon chain, such as oligohydroxy hexanes (e.g. 1,2- and 1,6-hexanediol or 1,2,6-hexanetriol), require at least three to four synthesis steps. Replacing this complex chemistry by a one-pot reaction via –C–O– bond cleavage from sugars would be a significant breakthrough for the use of renewable feedstocks. Cu is known for its dehydroxylation (deoxygenation) properties, yielding the desired products from sugars. In this joint research between academic and industrial chemistry, we have identified so far unknown intermediate products and present the first mechanism that explains the selective cleavage of OH-groups over copper. Strong interactions between polyols, unsaturated species and the copper surface are observed. Stable five-membered rings are formed with Cu via two vicinal OH-groups of the polyol reactant that makes these OH-groups inert to –C–O– bond cleavage. Adjacent free OH-groups in close proximity to the catalyst are dehydroxylated (deoxygenated). We further show that degradation of polyols not only occurs via commonly cited retro-aldol reactions. The formation of acid intermediates with subsequent decarboxylation is validated as a new pathway for –C–C– bond cleavage to short-chain polyols and CO2. The proposed mechanisms for –C–O– and –C–C– bond cleavage elucidate why hydrogenolysis reactions require high hydrogen pressure (up to 200 bar) to suppress the degradation of sugars and obtain high yields of deoxy C6 products. With this knowledge, the improvement of a standard commercial Cu-RANEY® catalyst under optimized reaction conditions was shown. In contrast to alumina-supported Cu, the Cu–Al alloy in a RANEY®-type catalyst shows selective –C–O– bond cleavage properties while maintaining the C6 carbon chain. These new insights into the transformation of sugars to value added commodities show the potential for new approaches in future biorefinery concepts.


 Please wait while we load your content...
Please wait while we load your content...