Conversion of lactide to acrylic acid by a phosphonium ionic liquid and acid cocatalyst†
Abstract
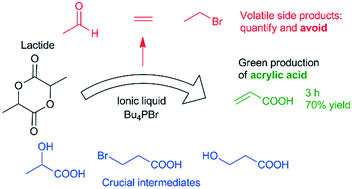
Acrylic acid, an important starting compound for a wide variety of consumer products, is generally produced through selective oxidation of propene. A more sustainable approach would be its synthesis from lactic acid, since this compound is generally produced by fermentation of renewable sugar streams. Up to now, most research on this reaction has involved gas phase reactions, which require high temperatures, often above 300 °C. Building on our previous work, which demonstrated the ionic liquid tetrabutylphosphonium bromide, combined with an acid cocatalyst, as an interesting catalyst for dehydration and dehydrohalogenation reactions, we here report the use of this catalytic system in the conversion of lactide to acrylic acid. Initial experiments showed the formation of the volatile side products ethylene, bromoethane and acetaldehyde, which could explain mass loss that was not well understood heretofore. A combination of liquid phase GC analysis and gas phase FTIR spectroscopy allowed us to close the mass balance. As a result, a reaction network was proposed and supported by experimental studies. Finally, the insights in the reaction network were applied to achieve a very good acrylic acid yield of 70%.


 Please wait while we load your content...
Please wait while we load your content...