Hydrotalcite based Ni–Fe/(Mg, Al)Ox catalysts for CO2 methanation – tailoring Fe content for improved CO dissociation, basicity, and particle size†
Abstract
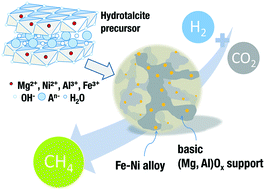
It is essential for mankind to address greenhouse gas emission, depletion of fossil resources and development of efficient storage and transportation of renewable energy. By using the atmospheric gas CO2 as raw material for the value-added chain, an overall CO2 neutral circular economy is imaginable. The power to gas (PtG) concept tackles all three topics by producing methane as energy carrier using renewable hydrogen and capture of CO2. Methanation has been known for decades; however, the preparation of noble metal free catalysts possessing high activity, selectivity and stability remains challenging. Bimetallic FexNix−1 alloy catalysts have shown synergistic effects for methanation as they can be tailored to the CO dissociation energy. Multicomponent hydrotalcite precursors allow close proximity of the individual metals enabling high dispersion and continuous variation of the composition. Herein, we studied Ni–Fe/(Mg, Al)Ox bimetallic catalysts derived via mixed hydrotalcite precursors. Iron has a significant influence on both activity and selectivity due to small particle sizes, facilitated CO dissociation, and tailored surface basicity. For the best catalyst (Fe/Ni = 0.1), the CO2 conversion rate reaches about 6.96 mmol CO2 molFe+Ni−1 s−1 at 335 °C with a selectivity of 99.3% to CH4, remaining constant for at least 24 h time on stream.


 Please wait while we load your content...
Please wait while we load your content...