How efficient is Li+ ion transport in solvate ionic liquids under anion-blocking conditions in a battery?†
Abstract
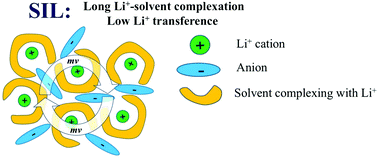
An experimental analysis based on very-low-frequency (VLF) impedance spectra and the Onsager reciprocal relations is combined with advanced analysis of dynamic correlations in atomistic molecular simulations in order to investigate Li+ transport in solvate ionic liquids (SILs). SILs comprised of an equimolar mixture of a lithium salt with glyme molecules are considered as a promising class of highly concentrated electrolytes for future Li-ion batteries. Both simulations and experiments on a prototypical Li-bis(trifluoromethanesulfonyl)imide (TFSI) salt/tetraglyme mixture show that while the ionic conductivity and the Li+ transport number are quite high, the Li+ transference number under ‘anion-blocking conditions’ is extremely low, making these electrolytes rather inefficient for battery applications. The contribution of cation–anion correlation to the total ionic conductivity has been extracted from both studies, revealing a highly positive contribution due to strongly anti-correlated cation–anion motions. Such cation–anion anti-correlations have also been found in standard ionic liquids and are a consequence of the constraint of momentum conservation. The molecular origin of low Li+ transference number and the influence of anti-correlated motions on Li+ transport efficiency have been investigated as a function of solvent composition. We demonstrate that Li+ transference number can be increased either by reducing the residence time between Li+ and solvent molecules or by adding excessive solvent molecules that are not complexing with Li+.


 Please wait while we load your content...
Please wait while we load your content...