The effect of N-methylation on the conformational landscape of alanine: the case of N-methyl-l-alanine†
Abstract
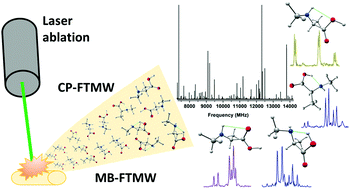
The non-proteinogenic amino acid N-methyl-L-alanine has been brought into the gas phase using laser ablation techniques and studied by high resolution chirped pulse and molecular-beam Fourier transform microwave spectroscopies coupled to supersonic expansion. Four conformers showing the three types of hydrogen bond interactions I (NH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), II (OH⋯N) and III (N–H⋯O–H) have been unambiguously identified, based on the comparison of the experimental rotational and 14N nuclear quadrupole constants with the calculated ab initio values. The observation of a type III conformer evidences the role of methyl groups in both sides to impose the steric hindrance, precluding the relaxation from type III to type I conformers and explains the responsibility for the unique conformational landscape observed in the case of NMA.
C), II (OH⋯N) and III (N–H⋯O–H) have been unambiguously identified, based on the comparison of the experimental rotational and 14N nuclear quadrupole constants with the calculated ab initio values. The observation of a type III conformer evidences the role of methyl groups in both sides to impose the steric hindrance, precluding the relaxation from type III to type I conformers and explains the responsibility for the unique conformational landscape observed in the case of NMA.


 Please wait while we load your content...
Please wait while we load your content...