Influence of the charge on the reactivity of azafullerenes†
Abstract
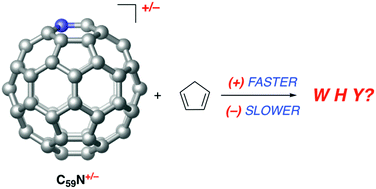
The influence of the charge on the Diels–Alder reactivity of azafullerenes (C59N+ and C59N−) has been computationally explored by means of density functional theory calculations. In addition, the regioselectivity of the process has been investigated and compared to the analogous cycloaddition reaction involving the parent neutral azahydro[60]fullerene C59NH. It is found that the [4+2]-cycloaddition reaction between C59N+ and cyclopentadiene, which occurs concertedly through a synchronous transition state, proceeds with a lower activation barrier and is more exothermic than the analogous process involving C59NH. In contrast, the anionic C59N− counterpart is clearly less reactive. This reactivity trend is quantitatively analyzed in detail by means of the activation strain model of reactivity in combination with the energy decomposition analysis method. It is found that the frontier molecular orbital interactions are not responsible for the observed reactivity trend but the Pauli repulsion between closed-shells mainly governs the transformation.


 Please wait while we load your content...
Please wait while we load your content...