Influence of argon and D2 tagging on the hydrogen bond network in Cs+(H2O)3; kinetic trapping below 40 K†
Abstract
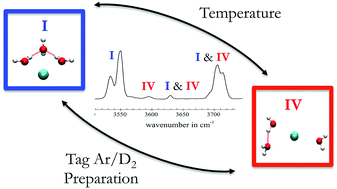
The influence of enthalpic and entropic effects as well as of kinetic trapping processes on the structure of Ar/D2-tagged Cs+(H2O)3 clusters is studied by temperature-dependent infrared photodissociation spectroscopy combined with harmonic vibrational spectra calculations and anharmonic free energy profiles from finite temperature metadynamics molecular dynamics simulations. Each tag favors a different hydrogen bond network of water molecules, with Ar-tagging (vs. D2-tagging) of Cs+(H2O)3 leading to the lower energy conformation. The relative population of these conformers can be tuned over a temperature range of 12 to 21 K. The formation mechanisms of these tagged clusters can be deduced from the free energy profiles. This investigation demonstrates that a variety of factors, both thermodynamic and kinetic, play a role in the structure of flexible molecular species, even at cryogenic temperatures.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...