Topologically close-packed characteristic of amorphous tantalum†
Abstract
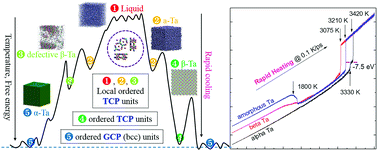
The structural evolution of tantalum (Ta) during rapid cooling was investigated by molecular dynamics simulation, in terms of the system energy, the pair distribution function and the largest standard cluster analysis. It was found that the critical cooling rate for vitrification was about R ≥ 0.25 K ps−1, two orders lower than other metals (such as Au, Ag, Al, Zr and Zn) and that the meta-stable σ phase (β-Ta) not only appears on the pathway from liquid to the stable body-centred cubic crystal, but is also easily obtained at room temperature as a long-lived metastable phase with some probability. The most interesting point is that the liquid, amorphous and β-Ta phases share a nontrivial structural homology; the intrinsic topologically close-packed (TCP) structures in liquids are inherited and developed in different ways, resulting in amorphous or crystalline solids, respectively. With highly local packing fractions and geometrical incompatibility with the global close-packed (such as hcp, fcc and bcc) crystals, TCP structures inevitably result in structural heterogeneity and favour vitrification. As a superset of icosahedrons, TCP structures are ubiquitous in metallic melts, and just before the onset of crystallization reach their maximal number, which is much bigger in Ta than in other poor-GFA metals; so we argue that the strong forming ability of TCP local structures significantly enhances the glass forming ability of pure metals. These findings open up a new perspective that could have a profound impact on the research into metallic glasses.


 Please wait while we load your content...
Please wait while we load your content...