Reversible hydrogen storage in pristine and Li decorated 2D boron hydride†
Abstract
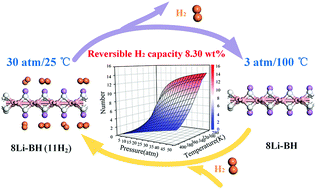
Motived by the recent experimental fabrication of two-dimensional boron hydride (BH) sheets (Nishino et al., J. Am. Chem. Soc. 2017, 139, 13761), we explore the feasibility of pristine and Li doped BH sheets as a hydrogen storage medium within the framework of density functional theory. BH shows an unexpected high affinity to Li with a binding energy of −2.38 eV in comparison to other alkali and alkaline earth metals (Na, K, Ca, Mg and Al), much larger than its bulk cohesive energy (−1.63 eV). Energy barriers of Li diffusion on BH are also determined to be around 1.12 eV, showing both high dynamic and thermodynamic stability without the issue of cluster formation. Moreover, Li decorated BH is expected to achieve a high theoretical gravimetric density of 11.57 wt% with an average H2 adsorption energy of −0.17 eV, holding great potential in massive hydrogen storage. In addition to the storage, thermodynamic analysis on the desorption behaviors of H2 molecules is performed via N–P–T diagram, which demonstrates that most of the H2 molecules (8.30 wt%) could be released at 3 atm/100 °C. Thus, the Li-decorated BH sheets are expected to be applied as an efficient medium for hydrogen storage under ambient conditions.


 Please wait while we load your content...
Please wait while we load your content...