Ion–peptide interactions between alkali metal ions and a termini-protected dipeptide: modeling a portion of the selectivity filter in K+ channels†
Abstract
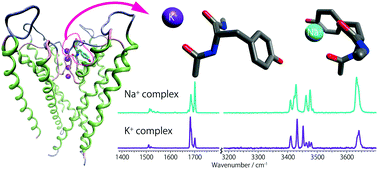
Potassium channels have the unique ability to allow the selective passage of potassium ions at near diffusion-free rates while inhibiting the passage of more abundant sodium ions. Local interactions between chemical functional groups and the ions are responsible for both selectivity and transport. As an initial step in characterizing these interactions, the structures of Na+ and K+ complexed to the Ac-Tyr-NHMe peptide have been determined from infrared laser spectroscopy and supporting ab initio calculations. Ac-Tyr-NHMe, a termini-protected peptide sequence, replicates the GYG portion of one of the four peptide chains comprising the selectivity filter of a K+ channel. This peptide contains two carbonyl groups, among the eight C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups forming the S1 binding site of the selectivity filter. Three conformations have been identified for both ions by laser IR–IR double resonance methods. Two conformations have the ion bound to the two C
O groups forming the S1 binding site of the selectivity filter. Three conformations have been identified for both ions by laser IR–IR double resonance methods. Two conformations have the ion bound to the two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. The third conformation has, in addition, a cation–π interaction with the aromatic ring of tyrosine, i.e. tridentate binding. The relative contributions of the three conformers are approximately the same for K+Ac-Tyr-NHMe, while the tridentate conformer is preferred for Na+Ac-Tyr-NHMe. These differences will be discussed in the context of ion mobility and selectivity.
O groups. The third conformation has, in addition, a cation–π interaction with the aromatic ring of tyrosine, i.e. tridentate binding. The relative contributions of the three conformers are approximately the same for K+Ac-Tyr-NHMe, while the tridentate conformer is preferred for Na+Ac-Tyr-NHMe. These differences will be discussed in the context of ion mobility and selectivity.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...