Solvation energies of the proton in methanol revisited and temperature effects†
Abstract
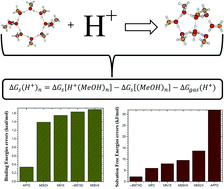
We report in this work the absolute solvation enthalpies and the absolute solvation free energies of the proton in methanol at temperatures ranging from 20 to 340 K and an extrapolation to a desired temperature. To achieve this, we thoroughly investigated the structures of neutral methanol clusters (MeOH)n=2–10 and those of the protonated methanol decamer H+(MeOH)n=10 at the M06-2X/6-31++g(d,p) level of theory. As a result, we noted that up to the octamer, the population of the neutral methanol clusters is constituted by cyclic isomers. For nonamers and decamers, both cyclic and branched cyclic isomers contribute to the population of the clusters. Moreover, folded or distorted cyclic isomers are the most favored at low temperatures, while higher temperatures favored the flat cyclic isomers for n = 7–9. For the methanol decamer, a branched cyclic isomer is found to be the most favored at low temperatures. Elsewhere, the infrared spectra of all the investigated structures are provided and compared against experiment. The binding energy of neutral methanol is calculated at the X/6-31++g(d,p) levels of theory, where X represents the DFT functionals M062X, APFD, MN15, ωB97XD and M08HX. It is observed that these functionals provide results in good agreement with the experimental vaporization enthalpy. However, the APFD functional shows the best performance followed by the other functionals in the order of M062X, MN15 and ωB97XD. Furthermore, the calculated solvation energies of the proton in methanol at these various levels of theory and at MP2/6-31++g(d,p) show that the ωB97XD functional shows the best performance in evaluating the solvation enthalpy and the solvation free energy of the proton in methanol and the calculated values are respectively −1140.5 kJ mol−1 and −1100.7 kJ mol−1 at room temperature. Elsewhere, we noted that the absolute solvation enthalpy of the proton in methanol is less affected by a change in temperature. However, the absolute solvation free energy of the proton in methanol remains constant only at temperatures lower than 180 K. For higher temperatures, the absolute solvation free energy of the proton in methanol increases as a linear function of the temperature and can be approximated by ΔGm(H+,T) = 0.200T − 1161.4.


 Please wait while we load your content...
Please wait while we load your content...