The role of hydrophobic, aromatic and electrostatic interactions between amino acid residues and a titanium dioxide surface†
Abstract
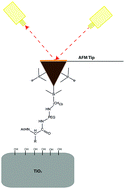
Understanding the nature of interactions between inorganic surfaces and biomolecules, such as amino acids and peptides, can enhance the development of new materials. Here, we present single molecule force spectroscopy (SMFS) measurements of the interactions between an atomic force microscopy (AFM) probe, modified with various amino acids, and a titanium dioxide surface. Specifically, we study the affinity of amino acids toward a titanium dioxide surface bearing hydrophobic (Leu), aromatic (Phe) and hydrophilic (Orn) residues. We find that aromatic interactions dominate over aliphatic in their affinity to the titanium dioxide surface. In addition, we show that by combining aromatic and hydrophilic moieties in a single amino acid (NH2-Phe), the adhesion of the latter to the surface increases. Furthermore, the affinity of positively charged amino acids to the titanium dioxide surface is higher than that of uncharged, and can be increased more, with elevating the pH of the buffer above the pKa of the basic residues. The kinetic and thermodynamic parameters imply that the dynamics of the surface–amino acid interface are mostly governed by hydrophobic interactions.


 Please wait while we load your content...
Please wait while we load your content...