The non-covalently bound SO⋯H2O system, including an interpretation of the differences between SO⋯H2O and O2⋯H2O†
Abstract
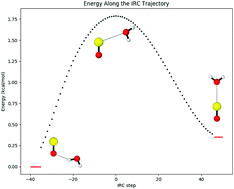
Despite the interest in sulfur monoxide (SO) among astrochemists, spectroscopists, inorganic chemists, and organic chemists, its interaction with water remains largely unexplored. We report the first high level theoretical geometries for the two minimum energy complexes formed by sulfur monoxide and water, and we report energies using basis sets as large as aug-cc-pV(Q+d)Z and correlation effects through perturbative quadruple excitations. One structure of SO⋯H2O is hydrogen bonded and the other chalcogen bonded. The hydrogen bonded complex has an electronic energy of −2.71 kcal mol−1 and a zero kelvin enthalpy of −1.67 kcal mol−1, while the chalcogen bonded complex has an electronic energy of −2.64 kcal mol−1 and a zero kelvin enthalpy of −2.00 kcal mol−1. We also report the transition state between the two structures, which lies below the SO⋯H2O dissociation limit, with an electronic energy of −1.26 kcal mol−1 and an enthalpy of −0.81 kcal mol−1. These features are much sharper than for the isovalent complex of O2 and H2O, which only possesses one weakly bound minimum, so we further analyze the structures with open-shell SAPT0. We find that the interactions between O2 and H2O are uniformly weak, but the SO⋯H2O complex surface is governed by the superior polarity and polarizability of SO, as well as the diffuse electron density provided by sulfur's extra valence shell.


 Please wait while we load your content...
Please wait while we load your content...