Atmospheric chemistry of iodine anions: elementary reactions of I−, IO−, and IO2− with ozone studied in the gas-phase at 300 K using an ion trap†
Abstract
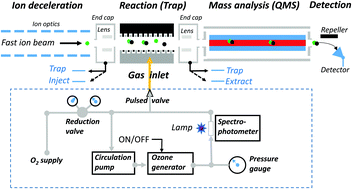
Using a radio-frequency ion trap to study ion–molecule reactions under isolated conditions, we report a direct experimental determination of reaction rate constants for the sequential oxidation of iodine anions by ozone at room temperature (300 K). The results are R1: I− + O3 → IO− + O2, k1 = (7 ± 2) × 10−12 cm3 s−1; R2: IO− + O3 → IO2− + O2, k2 = (10 ± 2) × 10−9 cm3 s−1; R3: IO2− + O3 → IO3− + O2, k3 = (16 ± 2) × 10−9 cm3 s−1. More oxidized forms such as IO4− and IO5− were not observed. Additionally, we performed quantum chemical calculations to elucidate the energetics of these oxidation reactions.


 Please wait while we load your content...
Please wait while we load your content...