Reactivity of the O2+·(H2O)n and NO+·(H2O)n cluster ions in the D-region of the ionosphere†
Abstract
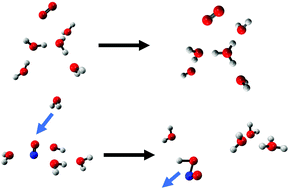
The protonated water clusters present in the D-region of the ionosphere have been postulated to be formed from cluster ions such as O2+·(H2O)n and NO+·(H2O)n, although the detailed mechanism of the underlying reactions is not understood. Second order Møller–Plesset perturbation theory based Born–Oppenheimer ab initio molecular dynamics (AIMD) simulations of the reactions of the O2+·(H2O)n and NO+·(H2O)n cluster ions to form protonated water clusters reveal different mechanisms for the O2+ and NO+ based ions. AIMD simulations of O2+·(H2O)n=2–5 with initial velocities of the atoms sampled from the Maxwell–Boltzmann distribution at 220 K show that following charge transfer, a reaction to form a protonated water cluster and OH occurs rapidly where the neutral O2 molecule is just a spectator. In contrast, the reaction of NO+·(H2O)n=4,5 has been hypothesised to involve an intracluster reaction, but no reaction is observed in AIMD simulations using thermal initial velocities. However, it is shown that reactions to form protonated water clusters do occur in simulations when a water molecule collides with a NO+·(H2O)4 cluster.


 Please wait while we load your content...
Please wait while we load your content...