Impact of surface wettability on dynamics of supercooled water confined in nitrogen-doped ordered mesoporous carbon†
Abstract
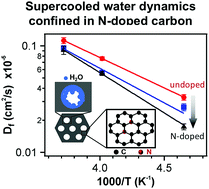
Confinement of water to nanoscale dimensions enables substantial supercooling through disruption of the hydrogen bonding network. However, there remain questions associated with the importance of the nature of the water–surface interactions relative to physical confinement defined by the pore geometry on the dynamics of supercooled water. Here, a simple route to tune the surface wetting properties through nitrogen doping of carbon is reported. This method leads to nearly indistinguishable mesopore sizes to enable separation of surface wettability and pore size effects. Quasielastic neutron scattering (QENS) is used to probe the proton dynamics of water confined within the mesopores with an average diameter of 4.85 ± 0.05 nm as a function of temperature from 267 K to 189 K. The motions of water in the mesopores follow jump-diffusion. For the temperatures examined, the diffusivity of water in the mesopores decreases with increasing nitrogen doping of the carbon framework. The activation energy associated with proton dynamics increases by approximately 30% with N-doping when compared to the undoped carbon surface, which is attributed to the enhanced surface wettability (favorable interactions between water and pore surface). This acts to provide an energy barrier for the water motions. This work suggests that the influence of surface chemistry on the dynamics of supercooled water confined in mesopores is less than the influence of nanopore size.


 Please wait while we load your content...
Please wait while we load your content...