Toward reliable characterization of energetic materials: interplay of theory and thermal analysis in the study of the thermal stability of tetranitroacetimidic acid (TNAA)†
Abstract
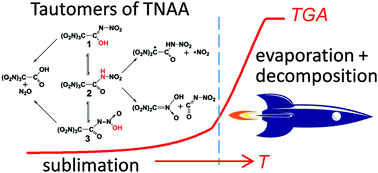
The thermal stability of energetic materials, being of the utmost importance for safety issues, is often considered in terms of kinetics, e.g., the Arrhenius parameters of the decomposition rate constant. The latter, in turn, are commonly determined using conventional thermoanalytical procedures with the use of simple Kissinger or Ozawa methods for kinetic data processing. However, thermal decomposition of energetic materials typically occurs via numerous exo- and endothermal processes including fast parallel reactions, phase transitions, autocatalysis, etc. This leads to numerous drawbacks of simple approaches. In this paper, we proposed a new methodology for characterization of the thermochemistry and thermal stability of melt-cast energetic materials, which is comprised of a complementary set of experimental and theoretical techniques in conjunction with a suitable kinetic model. With the aid of the proposed methodology, we studied in detail a novel green oxidizer, tetranitroacetimidic acid (TNAA). The experimental mass loss kinetics in the melt was perfectly fitted with a model comprised of zero-order reaction (sublimation or evaporation) and first-order thermal decomposition of TNAA with the effective Arrhenius parameters Ea = 41.0 ± 0.2 kcal mol−1 and log(A/s−1) = 20.2 ± 0.1. We rationalized the experimental findings on the basis of highly accurate CCSD(T)-F12 quantum chemical calculations. Computations predict that thermolysis of TNAA involves an intricate interplay of multiple decomposition channels of the three tautomers, which are equilibrated via either monomolecular reactions or concerted double hydrogen atom transfer in the H-bonded dimers; the calculated Arrhenius parameters of the effective rate constant coincide well with experiment. Most importantly, calculations provide detailed mechanistic evidence missing in the thermoanalytical experiment and explain formation of the experimentally observed primary products N2O and NO2. Along with the kinetics and mechanism of decomposition, the proposed approach yields accurate thermochemistry and phase change data of TNAA.


 Please wait while we load your content...
Please wait while we load your content...