The reaction of alkyl hydropersulfides (RSSH, R = CH3 and tBu) with H2S in the gas phase and in aqueous solution†
Abstract
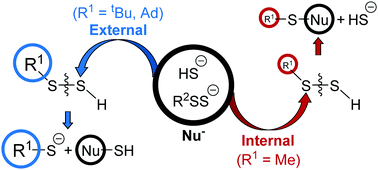
The RSSH + H2S → RSH + HSSH reaction has been suggested by numerous labs to be important in H2S-mediated biological processes. Seven different mechanisms for this reaction (R = CH3, as a model) have been studied using the DFT methods (M06-2X and ωB97X-D) with the Dunning aug-cc-pV(T+d)Z basis sets. The reaction of CH3SSH with gas phase H2S has a very high energy barrier (>45 kcal mol−1), consistent with the available experimental observations. A series of substitution reactions R1–S–S–H + −S–R2 (R1 = Me, tBu, Ad, R2 = H, S–Me, S–tBu, S–Ad) have been studied. The regioselectivity is largely affected by the steric bulkiness of R1, but is much less sensitive to R2. Thus, when R1 is Me, all −S–R2 favorably attack the internal S atom, leading to R1–S–S–R2. While for R1 = tBu, Ad, all −S–R2 significantly prefer to attack the external S atom to form −S–S–R2. These results are in good agreement with the experimental observations.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
