Gas–liquid phase equilibrium of a model Langmuir monolayer captured by a multiscale approach†
Abstract
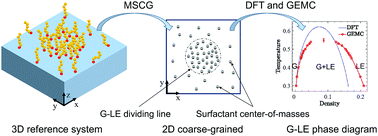
The gas–liquid expanded phase transition of a Langmuir monolayer happens at very low surface concentrations which makes this phenomenon extremely expensive to explore in finite three-dimensional (3D) atomistic simulations. Starting with a 3D model reference system of amphiphilic surfactants at a 2D vapor–liquid interface, we apply our recently developed approach (Phys. Chem. Chem. Phys., 2018, 20, 16238) and map the entire system to an effective 2D system of surfactant center-of-masses projected onto the interface plane. The coarse-grained interaction potential obtained via a force-matching scheme from the 3D simulations is then used to predict the 2D gas–liquid phase equilibrium of the corresponding Langmuir monolayer. Monte Carlo simulations in the Gibbs ensemble are performed to calculate areal densities, chemical potentials and surface pressures of the gaseous and liquid coexisting phases within the monolayer. We compare these simulations to the results of a 2D density functional approach based on Weeks–Chandler–Anderson perturbation theory. We furthermore use this approach to determine the density profiles across the equilibrium gas–liquid dividing line and the corresponding line tensions.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...