Influences of carboxyl functionalization of intercalators on exfoliation of graphite oxide: a molecular dynamics simulation†
Abstract
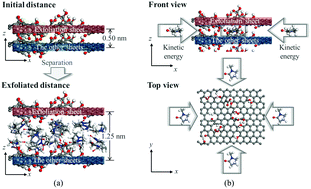
In this study, the influences of the carboxyl functionalization of intercalators on exfoliation of graphite oxide were analyzed using molecular dynamics (MD) simulations. Molecular models of four-layered graphene oxide (GO) sheets, four different solvents (ethanol, dimethylformamide, tetrahydrofuran, and N-methyl-2-pyrrolidone), and four different intercalators (anthracene, 2-anthracenecarboxylic acid, 2,3-anthracenedicarboxylic acid, and 2,6-anthracenedicarboxylic acid) were used in the MD simulations. A separation simulation of GO sheets was performed to determine the point at which the GO sheets begin to exfoliate. An insertion simulation was used to obtain the minimum kinetic energy required for exfoliation and to calculate GO–solvent and GO–intercalator interaction energies. As the simulation result, GO–solvent and GO–intercalator interactions affected the minimum kinetic energy required for exfoliation. Having more carboxyl functional groups on the anthracene improved both the GO–intercalator interaction and the efficiency of the intercalators during exfoliation. These results reveal that increasing the interaction energy between the GO sheets and the insertion molecules is an efficient way to improve the performance of the solvents and the intercalators for the exfoliation of GO sheets.


 Please wait while we load your content...
Please wait while we load your content...