Interfacial structural crossover and hydration thermodynamics of charged C60 in water†
Abstract
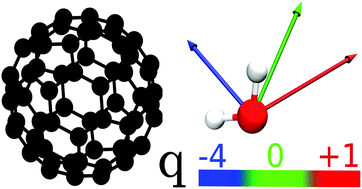
Classical molecular dynamics simulations of the hydration thermodynamics, structure, and dynamics of water in hydration shells of charged buckminsterfullerenes are presented in this study. Charging of fullerenes leads to a structural transition in the hydration shell, accompanied by creation of a significant population of dangling O–H bonds pointing toward the solute. In contrast to the well accepted structure–function paradigm, this interfacial structural transition causes nearly no effect on either the dynamics of hydration water or on the solvation thermodynamics. Linear response to the solute charge is maintained despite significant structural changes in the hydration shell, and solvation thermodynamic potentials are nearly insensitive to the altering structure. Only solvation heat capacities, which are higher thermodynamic derivatives of the solvation free energy, indicate some sensitivity to the local hydration structure. We have separated the solvation thermodynamic potentials into direct solute–solvent interactions and restructuring of the hydration shell and analyzed the relative contributions of electrostatic and nonpolar interactions to the solvation thermodynamics.


 Please wait while we load your content...
Please wait while we load your content...