Photoelectron spectroscopy and thermochemistry of o-, m-, and p-methylenephenoxide anions†
Abstract
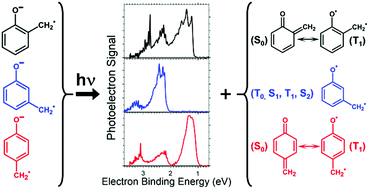
The anionic products following (H + H+) abstraction from o-, m-, and p-methylphenol (cresol) are investigated using flowing afterglow-selected ion flow tube (FA-SIFT) mass spectrometry and anion photoelectron spectroscopy (PES). The PES of the multiple anion isomers formed in this reaction are reported, including those for the most abundant isomers, o-, m- and p-methylenephenoxide distonic radical anions. The electron affinity (EA) of the ground triplet electronic state of neutral m-methylenephenoxyl diradical was measured to be 2.227 ± 0.008 eV. However, the ground singlet electronic states of o- and p-methylenephenoxyl were found to be significantly stabilized by their resonance forms as a substituted cyclohexadienone, resulting in measured EAs of 1.217 ± 0.012 and 1.096 ± 0.007 eV, respectively. Upon electron photodetachment, the resulting neutral molecules were shown to have Franck–Condon active ring distortion vibrational modes with measured frequencies of 570 ± 180 and 450 ± 80 cm−1 for the ortho and para isomers, respectively. Photodetachment to excited electronic states was also investigated for all isomers, where similar vibrational modes were found to be Franck–Condon active, and singlet–triplet splittings are reported. The thermochemistry of these molecules was investigated using FA-SIFT combined with the acid bracketing technique to yield 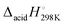 values of 341.4 ± 4.3, 349.1 ± 3.0, and 341.4 ± 4.3 kcal mol−1 for the o-, m-, and p-methylenephenol radicals, respectively. Construction of a thermodynamic cycle allowed for an experimental determination of the bond dissociation energy of the O–H bond of m-methylenephenol radical to be 86 ± 4 kcal mol−1, while this bond is significantly weaker for the ortho and para isomers at 55 ± 5 and 52 ± 5 kcal mol−1, respectively. Additional EAs and vibrational frequencies are reported for several methylphenyloxyl diradical isomers, the negative ions of which are also formed by the reaction of cresol with O−.
values of 341.4 ± 4.3, 349.1 ± 3.0, and 341.4 ± 4.3 kcal mol−1 for the o-, m-, and p-methylenephenol radicals, respectively. Construction of a thermodynamic cycle allowed for an experimental determination of the bond dissociation energy of the O–H bond of m-methylenephenol radical to be 86 ± 4 kcal mol−1, while this bond is significantly weaker for the ortho and para isomers at 55 ± 5 and 52 ± 5 kcal mol−1, respectively. Additional EAs and vibrational frequencies are reported for several methylphenyloxyl diradical isomers, the negative ions of which are also formed by the reaction of cresol with O−.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...