Elucidating the morphological aspects and proton dynamics in a hybrid perfluorosulfonic acid membrane for medium-temperature fuel cell applications†
Abstract
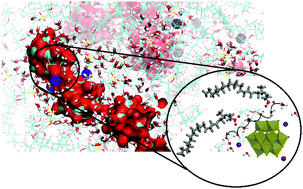
A perfluorosulfonic acid (PFSA) membrane, i.e. Nafion® 117, was doped with the heteropoly salts (HPS) Cs3PW12O40, Rb3PW12O40, and (NH4)3PW12O40. Also, composite membranes with CsxH3−xPW12O40 (x = 1, 2, and 3) as dopants were investigated, which were rendered insoluble by substituting protons with larger cations. Morphological assessment and a detailed analysis of the hopping events via SAXS measurement and analysis of the hydrogen bond networks were performed using classical and quantum hopping molecular dynamics simulation. The phase segregation decreased by increasing the extent proton substitution in HPA. HPS containing cations with a larger ionic radius induced smaller phase segregation in the membrane, as confirmed by the RDF plots. SAXS simulation revealed that the hydrophilic phase domains in the HPS-doped Nafion® membrane were spaced further apart than that in the HPA-doped membrane. Although there was a greater number of isolated clusters for the Cs3PW12O40-doped Nafion®, the average number of cluster decreased with an increase in the substitution cation/proton ratio and ionic radius of the cation. The analysis of the H-bond network stability revealed that the proton hops slower when the membrane contains HPS particles and the mean residence time of a proton on water molecules increases with an increase in the extent of proton substitution in H3PW12O40. Indeed, for the HPA-doped membrane, the diffusion of water molecules is lower than that in the HPS-doped system.


 Please wait while we load your content...
Please wait while we load your content...