Ligand field effects on the ground and excited states of reactive FeO2+ species†
Abstract
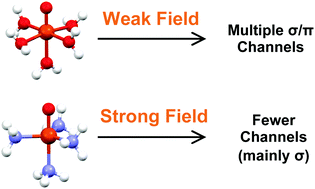
High-valent Fe(IV)-oxo species have been found to be key oxidizing intermediates in the mechanisms of mononuclear iron heme and non-heme enzymes that can functionalize strong C–H bonds. Biomimetic Fe(IV)-oxo molecular complexes have been successfully synthesized and characterized, but their catalytic reactivity is typically lower than that of the enzymatic analogues. The C–H activation step proceeds through two competitive mechanisms, named σ- and π-channels. We have performed high-level wave function theory calculations on bare FeO2+ and a series of non-heme Fe(IV)-oxo model complexes in order to elucidate the electronic properties and the ligand field effects on those channels. Our results suggest that a coordination environment formed by a weak field gives access to both competitive channels, yielding more reactive Fe(IV)-oxo sites. In contrast, a strong ligand environment stabilizes only the σ-channel. Our concluding remarks will aid the derivation of new structure–reactivity descriptors that can contribute to the development of the next generation of functional catalysts.


 Please wait while we load your content...
Please wait while we load your content...