What kind of neutral halogen bonds can be modulated by solvent effects?†
Abstract
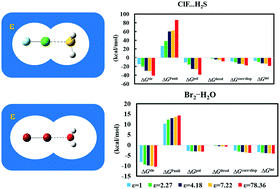
The response of a series of neutral halogen bonds X′–X⋯Y (X′–X = BrF, ClF, I2, Br2 and Cl2; Y = pyridine, NH3, H2S, HCN, H2O and dimethyl ether) to solvent effects is investigated using quantum theory of atoms in molecules (AIM), molecular electrostatic potential (MESP) and generalized Kohn–Sham energy decomposition analysis (GKS-EDA). The physical origin of the halogen bonds in various environments is explored. It is shown that halogen bonds in the gas phase are indeed governed by electrostatic interactions. A linear correlation between the magnitude of the σ hole and the electrostatic interaction is established. If the local softness of the donor or the acceptor is large, the polarization of the corresponding XB complex is large. Otherwise, the polarization is small. From gas phase to solvent environments, polarization is more sensitive to the solvent effects than the other interaction terms. For the strong XBs in a polar solvent environment, polarization is even larger than the electrostatic interaction. Our study shows that a halogen bond with a large portion of polarization can be modulated by solvent effects. If the contribution of polarization is small, the corresponding halogen bond is insensitive to solvent effects.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...