Water phase transitions from the perspective of hydrogen-bond network analysis
Abstract
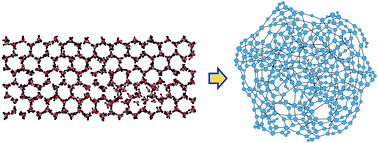
We analyze the water vapour–liquid and solid–liquid phase transitions from the perspective of hydrogen bond networks. Using molecular dynamics simulation data for the TIP4P/2005 and TIP4P/ice water models, we built hydrogen bond networks in the neighbourhood of the transitions. We studied the behaviour of some topological network properties: the average degree, clustering coefficient, and average path length. We found that these properties exhibit a discontinuity while approaching a phase transition region, similar to those that appear for some thermodynamic properties in the same region. This approach can be extended to characterize other water phase transitions. Besides, it can also be applied to study the phase transitions of other hydrogen-bonded substances or to other scenarios whose relevant “interaction” could be identified together with a “proper criterion” defined in an analogous way as in the case of hydrogen bonded systems.
- This article is part of the themed collection: Celebrating recent chemical science in Mexico


 Please wait while we load your content...
Please wait while we load your content...