How a linear triazene photoisomerizes in a volume-conserving fashion†
Abstract
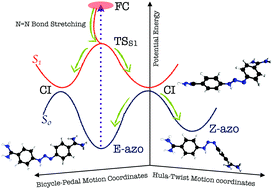
Understanding deactivation mechanisms of functional groups is a key step to design novel photo-active devices and molecular imaging agents. Here, we elucidate the photochemistry of linear triazenes, an extended analogue of the photo-switchable azo group, exemplarily for the widely used DNA-minor-groove binder berenil. Combining ultrafast spectroscopy and ab initio calculations unveils that the E-azo,s-trans structure of berenil predominates in the gas phase and in aqueous solution, and ADC(2) intrinsic reaction coordinate calculations disclose that the excited-state relaxation to the S1 minima/conical intersections follows a two-step mechanism: N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond stretching followed by a bicycle-pedal rotation in the triazene bridge. Furthermore, studying the ground-state pathways shows that a fraction of the molecules relaxes back to the E-azo,s-trans isomer while the other part photoisomerizes to the Z-azo,s-trans via a hula-twist motion, as evidenced by experimental quantum yields of Φ ≈ 0.5 found for berenil in water, ethylene glycol, or bound to β-trypsin. Moreover, our studies show that while the excited-state relaxation is insensitive to the environment, the ground-state dynamics depend on biomolecular binding partners.
N bond stretching followed by a bicycle-pedal rotation in the triazene bridge. Furthermore, studying the ground-state pathways shows that a fraction of the molecules relaxes back to the E-azo,s-trans isomer while the other part photoisomerizes to the Z-azo,s-trans via a hula-twist motion, as evidenced by experimental quantum yields of Φ ≈ 0.5 found for berenil in water, ethylene glycol, or bound to β-trypsin. Moreover, our studies show that while the excited-state relaxation is insensitive to the environment, the ground-state dynamics depend on biomolecular binding partners.


 Please wait while we load your content...
Please wait while we load your content...