Quantifying the protein–protein association rate in polymer solutions: crowding-induced diffusion and energy modifications
Abstract
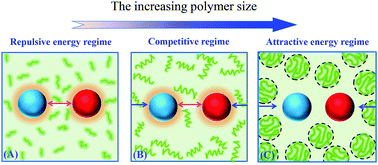
A theoretical framework is developed to study protein–protein association in polymer solutions under diffusion-limited conditions. Starting from the basal association rate, two fundamental aspects concerning macromolecular crowding are particularly taken into account. One is the effect of microviscosity on protein diffusion. The length-scale dependent relations of translational and rotational diffusion coefficients are incorporated. Another is relevant to the crowding-induced effective interaction between the pair of proteins. The resultant energy modifications to the basal association rate are properly introduced, following an instructive classification with increasing crowder size: repulsive interaction dominant, repulsive and attractive interactions competitive, and attractive interaction prevailing. With specific energy modification terms, we are able to investigate the deviations of the association rate from the Stokes–Einstein (SE) behavior (i.e., the linear relationship with respect to the reciprocal of macroviscosity) in a quantitative manner. Our theory is applied to study the association of TEM1–β-lactamase (TEM) and the β-lactamase inhibitor protein (BLIP) in polyethylene glycol (PEG) solutions, with varying concentration and polymerization. We explicitly evaluate the relative association rate constant as a function of the solution macroviscosity. The theoretical results demonstrate very good agreement with the experimental data. Moreover, the complicated non-trivial deviations, either positive (slower than SE) or negative (faster than SE), are systematically rationalized. The precise role of energy modifications and the microviscosity effect on diffusion, in particular on rotational diffusion, are clearly unraveled.


 Please wait while we load your content...
Please wait while we load your content...