Aggregation response of triglyceride hydrolysis products in cyclohexane and triolein†
Abstract
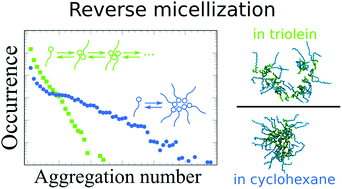
Here, we examine the aggregation response of a series of triglyceride-based biosurfactants in cyclohexane and triglyceride solvents via all-atom molecular dynamics simulations and supporting experiments. The surfactant aggregation follows in all systems, with only minor deviations, a multiple equilibrium, i.e. open association, model. Monoglyceride aggregation in cyclohexane exhibits a critical micellization concentration, cmc, showing a cmc can exist even in a system following open association. However, the cmc is associated with a change in balance with oligomeric and larger aggregates in the solution, not an onset of aggregate formation. It is demonstrated that reverse micelles can form in the absence of water stabilized by intersurfactant hydrogen bonds alone, and that the polarity and hydrogen bonding capability of triolein systematically reduces surfactant aggregation in comparison to cyclohexane. A comparison between CHARMM27 and CHARMM36 simulation models reveals that while trends are preserved, the models differ in quantitative prediction. Finally, consolidation of the general aggregation response trends predicted by the modelling are obtained via 7,7,8,8-tetracyanoquinodimethane dye (TCNQ) solubilization experiments on the corresponding model plant oil systems. The findings provide guidelines for predicting and controlling surfactant aggregation response in organic solvents via tuning the solvent polarity and hydrogen bonding ability, and a critical assessment of simulation and aggregation models for surfactant systems in organic solvents.


 Please wait while we load your content...
Please wait while we load your content...