Spin-paired solvated electron couples in alkali–ammonia systems†
Abstract
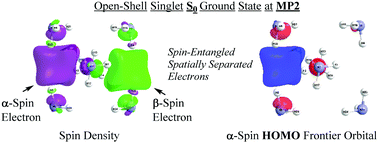
Alkali metals form deep blue solutions of solvated electrons in liquid ammonia. To explain the diamagnetism of more concentrated solutions, DFT and ab initio computations have been used to show that spin-paired couples of electrons can overcome Coulomb repulsion, occupying a cavity formed by solvent molecules, comparable in size to that of single solvated electrons. Sluggish hydrogen evolution from the solutions is rationalized by a high activation barrier for reaction of the electron pair with an ammonia molecule.


 Please wait while we load your content...
Please wait while we load your content...