Reactive molecular dynamics simulation of thermal decomposition for nano-aluminized explosives†
Abstract
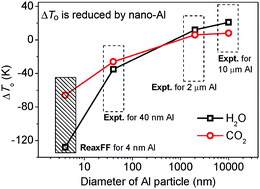
Aluminized explosives have important applications in civil construction and military armaments, but their thermal decomposition mechanisms are not well characterized. Here, the thermal decomposition of TNT, RDX, HMX and CL-20 on Al nanoparticles is examined by reactive dynamics simulations using a newly parameterized reactive force field with low gradient correction (ReaxFF-lg). Partially passivated Al nanoparticles were constructed and mixed with TNT, RDX, HMX and CL-20 crystals and then the mixed systems are heated to a high temperature in which the explosives are fully decomposed. The simulation results show that the aluminized explosives undergo three main steps of thermal decomposition, which were denoted “adsorption period” (0–20 ps), “diffusion period” (20–80 ps) and “formation period” (80–210 ps). These stages in sequence are the chemical adsorption between Al and surrounding explosive molecules (R–NO2–Al bonding), the decomposition of the explosives and the diffusion of O atoms into the Al nanoparticles, and the formation of final products. In the first stage, the Al nanoparticles decrease the decomposition reaction barriers of RDX (1.90 kJ g−1), HMX (1.95 kJ g−1) and CL-20 (1.18 kJ g−1), respectively, and decrease the decomposition reaction barrier of TNT from 2.99 to 0.29 kJ g−1. Comparing with the crystalline RDX, HMX and CL-20, the energy releases are increased by 4.73–4.96 kJ g−1 in the second stage. The number of produced H2O molecules increased by 25.27–27.81% and the number of CO2 molecules decreased by 47.73–68.01% in the third stage. These three stages are further confirmed by the evolutive diagram of the structure and temperature distribution for the CL-20/Al system. The onset temperatures (To) of generating H2O for all the aluminized explosives decrease, while those of generating CO2 for aluminized HMX and CL-20 increase, which are in accord with the experiment of aluminized RDX.


 Please wait while we load your content...
Please wait while we load your content...