Base adsorption mechanism over zeolite catalysts at different Al contents probed by the tapered element oscillating microbalance (TEOM)†
Abstract
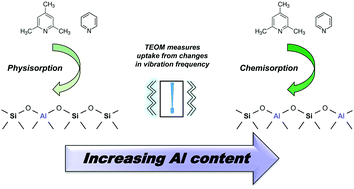
Acidity of zeolites is a paramount property that determines their behaviour in catalytic and adsorption applications. Various techniques have been established over the years to characterise this property qualitatively and quantitatively, using different indicators able to reveal different insights. In this work, for the first time we have used the tapered element oscillating microbalance (TEOM) to study the quantitative aspects of the internal and external acidity of zeolites by measuring the sorption dynamics of pyridine and collidine over HZSM5 zeolites with different silica-to-alumina ratios (SAR). The method is able to easily quantify, with a rapid and robust calibration procedure, maximum, physisorbed and chemisorbed uptake of probe molecules. The results show that the uptake of both pyridine and collidine measured by the TEOM increases with decreasing SAR, that is, with the increase in Al content, consistent with the increase in acid site density at increasing Al content. Most importantly, by providing a robust and easy-to-interpret set of data, the experimental protocol reveals new fundamental insights into the adsorption mechanism as a function of the Al content, showing that at high Al content chemisorption is the major adsorption mechanism, whereas at low Al content physisorption becomes the dominant mechanism.


 Please wait while we load your content...
Please wait while we load your content...