Electronic structure of the para-dinitrobenzene radical anion: a combined 2D photoelectron imaging and computational study†
Abstract
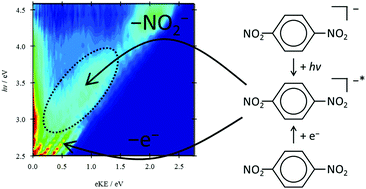
The para-dinitrobenzene radical anion has been studied by 2D photoelectron imaging within the energy range of 2.5 eV above the detachment threshold. Supporting electronic structure calculations at the XMCQDPT2 level of the excited states and resonances are presented. The direct photodetachment channel has been observed and modelled, and yields an electron affinity of 1.99 ± 0.01 eV. In addition to the direct channel, evidence of resonances is observed. These resonances, which are symmetry allowed for photoexcitation from the ground state and of Feshbach types with respect to the open continuum, result in fast internal conversion to bound electronic states, followed by statistical electron emission observed at very low kinetic energies as well as dissociation of the nitrite anion. The latter is seen in the photoelectron spectra, which can be modelled as a combination of direct detachment from the para-dinitrobenzene and nitrite anions. An additional dimension has been offered by the 2D photoelectron angular distribution that is particularly sensitive to a mechanism of electron detachment, allowing us to confidently interpret the production of the nitrite anion photofragment.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...