Self-inhibition of insulin amyloid-like aggregation†
Abstract
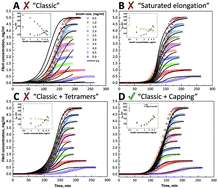
Protein misfolding and amyloid formation are related to multiple diseases. Besides its relation to injection-localized amyloidosis, insulin is also often used as a model protein to study amyloid aggregation in vitro. Possible mechanisms for aggregation of insulin monomers into amyloid-like fibrils are described in several publications, but the role of native-like oligomers, which are present in solution above pH 2, is poorly understood. Here we show that the addition of sodium chloride shifts the equilibrium from monomers towards oligomers without affecting the secondary structure of insulin. Initial analysis of the aggregation kinetics showed unusual dependence of aggregation half-times on the initial insulin concentration, suggesting the possibility of self-inhibition. Global fitting of the kinetic data revealed possible capping of fibril ends by insulin tetramers, leading to the inhibition of fibril elongation.


 Please wait while we load your content...
Please wait while we load your content...