Amide–imide tautomerization in the glutamine side chain in enzymatic and photochemical reactions in proteins†
Abstract
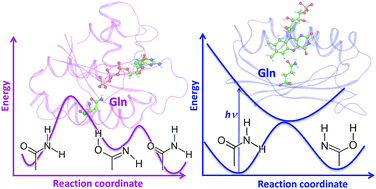
Amide–imide tautomerization presents a pervasive class of chemical transformations in organic chemistry of natural compounds. In this Perspective, we describe two distinctively different protein systems, in which the amide–imide tautomerization in the glutamine side chain takes place in enzymatic or photochemical reactions. First, hydrolysis of guanosine triphosphate (GTP) catalyzed by the Ras-GAP protein complex suggests the occurrence of the imide tautomer of glutamine in reaction intermediates. Second, photoexcitation of flavin-binding protein domains (BLUFs) initiates a chain of reactions in the chromophore-binding pocket, including amide–imide tautomerization of glutamine. Mechanisms of these reactions at the atomic level have been revealed in quantum mechanics/molecular mechanics (QM/MM) simulations. To reinforce conclusions on the critical role of amide–imide tautomerization of glutamine in these reactions we describe results of new quantum chemistry and QM/MM calculations for relevant molecular model systems. We reexamine results of the recent IR spectroscopy studies of BLUF domains, which provide experimental evidences of Gln tautomerization in proteins. We also propose to validate the glutamine-assisted mechanism of enzymatic GTP hydrolysis by using IR spectroscopy in a proper range of wavenumbers.
- This article is part of the themed collection: PCCP Perspectives


 Please wait while we load your content...
Please wait while we load your content...