Synergistic oxygen reduction of dual redox catalysts boosting the power of lithium–air battery†
Abstract
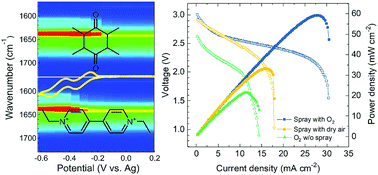
The development of rechargeable Li–air batteries has been confronted by the critical challenges of large overpotential loss, low achievable capacity, and prohibitively poor cycling and power performance. Surface passivation and pore clogging of the cathode due to the formation of Li2O2 during discharge result in sluggish interfacial charge transfer and have an impact on the mass transport of Li+ ions and O2 in the electrode, consequently giving rise to large voltage hysteresis and premature termination of discharge with low power performance. Here we report a redox flow lithium–oxygen cell with a modified redox electrolyte to tackle these issues. With the assistance of redox mediators, the cell presents substantially enhanced power performance in O2 and dry air during discharge. Through in situ spectroelectrochemical measurements and theoretical calculations, an oxygen reduction intermediate was unequivocally identified. By judiciously optimizing the redox electrolyte, the cell operates at near complete utilization of Li metal upon multiple refueling. The redox flow lithium–oxygen cell demonstrated here is envisaged to provide a pragmatic approach for the implementation of lithium–oxygen battery chemistry and to pave the way for advanced large-scale energy storage.


 Please wait while we load your content...
Please wait while we load your content...