Suppression by Pt of CO adsorption and dissociation and methane formation on Fe5C2(100) surfaces†
Abstract
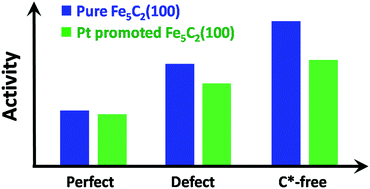
To understand the chemical origin of platinum promotion effects on iron based Fischer–Tropsch synthesis catalysts, the effects of Pt on CO adsorption and dissociation as well as surface carbon hydrogenation on the Fe5C2(100) facet with different surface C* contents have been studied using the spin-polarized density functional theory method. CO dissociation initiating from diverse sites was calculated through both direct and H-assisted pathways via the CHO intermediate. On the perfect (100) surface, CO can hardly dissociate, and the surface carbon can be facially hydrogenated to CH4. On the C*-defect and C*-free (100) surfaces, CO can strongly adsorb on the C* vacant sites and direct dissociation is favored to occur. The activity is higher with the decrease of the surface carbon content. When platinum atoms are added on the surfaces, the C*-vacancies have a higher activity for CO dissociation than the new sites generated by Pt adsorption. However, both the CO dissociation and the surface carbon consumption through CH4 formation are hindered. The evolution of surface carbon is predicted to be suppressed by the addition of Pt on the Fe5C2(100) surface.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...