Molecular thermodynamic modeling of a bilayer perforation in mixed catanionic surfactant systems†
Abstract
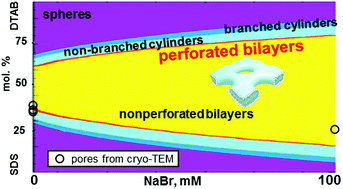
Perforated bilayers play an essential role in biology and in surface science. Here, we extend the classical aggregation model of catanionic surfactant mixtures to describe perforations in a self-assembled bilayer in aqueous salt. The model predicts that changing solution salinity and anionic-to-cationic surfactant ratio may lead to the spontaneous formation of pores in the bilayer and to the assembly of a micellar network. We estimate the dimensions of an optimal pore as a function of solution salinity and aggregate composition and show that with an increase of concentration of the deficient surfactant in a catanionic mixture, both the diameter and the thickness of the optimal pore decrease. This decrease is stronger for pores enriched in surfactant having a longer tail than for the pores enriched in the oppositely charged surfactant with a shorter tail. Our model helps to quantify the driving forces for the formation of a pore in a catanionic bilayer and to understand its role. For the aqueous mixtures C16TAB/SOS/NaBr and DTAB/SDS/NaBr, our predictions are in reasonable although not quantitative agreement with available cryo-TEM and SANS data. Predicted radii of perforations are in the range of those obtained from SANS data for perforated bilayer disks.


 Please wait while we load your content...
Please wait while we load your content...