Tropospheric oxidation of methyl hydrotrioxide (CH3OOOH) by hydroxyl radical†
Abstract
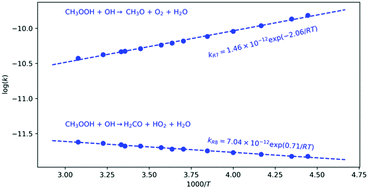
We have employed high level theoretical methods to investigate the oxidation of methyl hydrotrioxide by hydroxyl radical, which is of interest in atmospheric chemistry research. The reaction can proceed by abstraction of either the terminal hydrogen atom of OOH group producing CH3O, O2 and H2O, or one hydrogen atom of the CH3 group forming H2CO, HO2 and H2O. The rate constants for both reactions at 298 K are computed to be 4.7 × 10−11 and 2.1 × 10−12 cm3 molecule−1 s−1, respectively, that is, the abstraction of terminal hydrogen atom of the OOH group is about 22 times faster than that of a hydrogen atom of the CH3 group. The rate constant for the overall CH3OOOH + OH reaction is computed to be 4.9 × 10−11 cm3 molecule−1 s−1. Our calculations predict branching ratios between 99.0 and 93.9% for the formation of methoxy radical plus molecular oxygen and water, and between 1.0 and 6.1% for the formation of formaldehyde plus hydroperoxyl radical and water, in the 225–325 K temperature range. The lifetime of CH3OOOH in the troposphere is predicted to range from of 1.8 hours at 225 K, up to 3.9 hours at 275 K and decreasing to 0.2 hours at 310 K. CH3OOO and CH2OOOH radicals have been also investigated.


 Please wait while we load your content...
Please wait while we load your content...