Ostwald's rule of stages and metastable transitions in the hydrogen–water system at high pressure†
Abstract
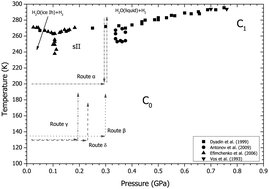
Although the hydrogenous analogue of the D2–D2O system has been well explored in the regimes above 1 GPa, and below 0.2 GPa, there have been very few studies in the region between these pressures. The recent discovery in the range 0.5–0.7 GPa of a new phase, C0, that possesses a new clathrate structure with a new H2O network, along with the proposal of another structure stable at similar conditions, has prompted further studies of the hydrogen water system in this intermediate pressure region. Here, we report the results of neutron-diffraction experiments that observed transitions from metastable to stable structures in the D2–D2O system around 0.2–0.3 GPa between 130 K and 280 K. These metastable structures were observed in the stability region of the sII hydrogen hydrate clathrate and computational studies of their relative enthalpies suggest that transition sequence observed is in line with Ostwald's ‘Rule of Stages’.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...