C–O bond activation and splitting behaviours of CO2 on a 4H-SiC surface: a DFT study†
Abstract
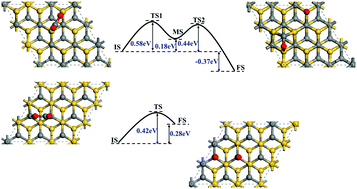
Conversion of CO2 into valuable chemicals can not only reduce the amount of CO2 in the atmosphere, but also realize the reuse of resources. It's well known that C–O bond activation and splitting are critical steps in the CO2 conversion process and it's crucial to employ an appropriate catalyst. Here, the adsorption and activation behaviors of a CO2 molecule on 4H-SiC surfaces were systematically investigated based on DFT calculations. Calculation results show that the CO2 molecule can anchor on 4H-SiC(0001) and (000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) surfaces. On the 4H-SiC(0001) surface, the adsorbed CO2 molecule prefers to dissociate with an energy barrier of 0.52–0.70 eV, producing an O adatom and a CO molecule on the surface. Further dissociation of the CO is hindered due to a large energy barrier of 2.12 eV. However, if a H atom is introduced, the CO molecule may combine with H into a CHO group and the reaction energy barrier is 1.69 eV. Moreover, the CHO group tends to transform into a CH group and an O adatom, a reaction in which a relatively low energy barrier of 0.09 eV needs to be surmounted. For the 4H-SiC(000
) surfaces. On the 4H-SiC(0001) surface, the adsorbed CO2 molecule prefers to dissociate with an energy barrier of 0.52–0.70 eV, producing an O adatom and a CO molecule on the surface. Further dissociation of the CO is hindered due to a large energy barrier of 2.12 eV. However, if a H atom is introduced, the CO molecule may combine with H into a CHO group and the reaction energy barrier is 1.69 eV. Moreover, the CHO group tends to transform into a CH group and an O adatom, a reaction in which a relatively low energy barrier of 0.09 eV needs to be surmounted. For the 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) case, the direct C–O bond dissociation energy barrier for CO2 is only 0.37 eV while further breaking of the C–O bond in CO is energetically unfavorable even with the help of a H atom. So the final products are an O adatom and CO chemisorbed on the 4H-SiC(000
) case, the direct C–O bond dissociation energy barrier for CO2 is only 0.37 eV while further breaking of the C–O bond in CO is energetically unfavorable even with the help of a H atom. So the final products are an O adatom and CO chemisorbed on the 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) surface. All the calculation results demonstrate that the inert CO2 molecule can be effectively activated on both the 4H-SiC(0001) and (000
) surface. All the calculation results demonstrate that the inert CO2 molecule can be effectively activated on both the 4H-SiC(0001) and (000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) surfaces and different splitting products could be obtained on the two different surfaces, implying that SiC is an applicable catalyst material for CO2 conversion with high efficiency and product selectivity.
) surfaces and different splitting products could be obtained on the two different surfaces, implying that SiC is an applicable catalyst material for CO2 conversion with high efficiency and product selectivity.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...