Counteraction of denaturant-induced protein unfolding is a general property of stabilizing agents†
Abstract
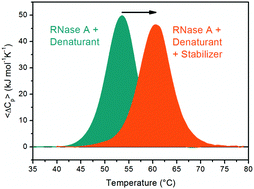
DSC measurements on RNase A at neutral pH show that five stabilizing agents, namely trimethylamine N-oxide, glucose, sucrose, betaine and sodium sulfate, can counteract the destabilizing action of urea, sodium perchlorate, guanidinium chloride and guanidinium thiocyanate. This is an important finding inferring that counteraction has a common physical origin, regardless of the chemical differences among the stabilizing agents and among the destabilizing ones. A rationalization is provided grounded on the following line of reasoning: (a) the decrease in solvent-excluded volume effect is the main stabilizing contribution of the native state; (b) its magnitude increases on increasing the density of the aqueous solution; (c) the density increases significantly in the ternary solutions containing water, a stabilizing agent and a destabilizing one, as indicated by the present experimental data.


 Please wait while we load your content...
Please wait while we load your content...