Explaining urokinase type plasminogen activator inhibition by amino-5-hydroxybenzimidazole and two naphthamidine-based compounds through quantum biochemistry†
Abstract
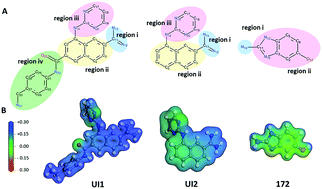
Urokinase plasminogen activator (uPA) is a biomarker and therapeutic target for several cancer types whose inhibition has been shown to slow tumor growth and metastasis. In this work, crystallographic data of uPA complexed with distinct ligands (PDB id: 1SQA, 1SQO, and 1FV9) were used to perform quantum biochemistry calculations based on the framework of density functional theory (DFT) and within the molecular fractionation with conjugated caps (MFCC) scheme. Our calculations revealed a total energy interaction of −107.30, −99.5, and −35.30 kcal mol−1 for two naphthamidine-based compounds (Ul1 and UI2) and 2-amino-5-hydroxybenzimidazole (172), respectively, which are in good agreement with known inhibitory experiments. Residues Asp189, Ser190, Cys191–Cys220, Gln192, Trp 215, Gly216, and Gly219 were identified as the main interacting amino acid residues with interaction energy contributions lower than −4.0 kcal mol−1 for uPA/UI1 and UPA/UI2 complexes. In the case of compound 172, our calculations have shown that the most important interactions occur with residues Asp189, Cys191–Cys220, and Ser190. Our results highlight the relevance of the protonation state of ligands and residues and that the naphthamidine scaffold of UI1 and UI2 is the main determinant of their potency, followed by their aminopyrimidine substitution. Altogether, the results of this work contribute to the understanding of the uPA binding mechanisms of the inhibitory compounds Ul1 and 172, stimulating the use of quantum biochemistry theoretical approaches for the development of new uPA inhibitors as new medicines for cancer treatment.


 Please wait while we load your content...
Please wait while we load your content...